
NEWSLETTER
ISSUE
Jul to Sep, 2019 Volume 9
Importance of Cleaning in Pharmaceutical processing
What is Cleaning
Cleaning is a procedure used for removal of product residues, degradation products, preservatives, excipients, and/or cleaning agents as well as the control of potential microbial contaminants. The results should ensure a). Removal of all residue of soil & washing agent, b) Chemically clean, c) meet product integrity requirement and d) to comply with regulatory requirements.
Why is Cleaning important
Cleaning is a critical process in Pharmaceutical manufacturing. If not performed well, it may end in cross-contamination with the subsequent batch, which may result in increased risk to consumer safety. Cleaning helps maintain product quality by preventing the transfer of ingredient, impurity from one batch to another or one product to another. Cleaning helps in the effective sanitisation of equipment surfaces. It ensures consumer safety, complying to regulatory standards. Moreover, it improves productivity and enhances user safety by providing a clean working environment and smooth functioning of equipment.
Generally, human error is assumed as cause of non-conformity in the cleaning process. However, it is essential to note that failure of cleaning process is not a human error but failure to recognize ‘Cleaning’ as a formal process and treat accordingly.
As per the FDA’s cGMP regulations, the entire cleaning process must be standardised and documented. As per regulation, Pharmaceutical manufacturers are required to set up a fully documented & written cleaning procedure for each piece of process equipment in compliance with FDA 21 CFR part 211.67.
How is Cleaning done

Cleaning pharmaceutical process equipment is challenging as each machine is designed differently for different purposes. Some of the commonly used machines in Pharmaceutical industry is process tanks, mixing tanks or reactors, centrifuges, filters, conveyors, granulators, filling machines, tabletting machines, glassware, containers and other laboratory items. Therefore, the Cleaning process needs to be customised for each application. However, there are various other factors such as qty and type of soil present, type of manufacturing equipment, cleaning method, surfaces to be cleaned, selection of cleaning detergent and temperature that should be considered while establishing cleaning procedure.
An effective cleaning process is defined, taking into consideration factors that are based on detailed knowledge of product mix & cleaning chemistry. The process can be developed effectively in close cooperation with the user.
A typical program of effective cleaning process development consists of
- Definition of cleaning task
- Technical consideration
- Evaluation of cleaning process
- Definition of the optimum cleaning process
- Verification of performance at the production site
- Analysis for possible residues
- Completion of cleaning validation
- Comprehensive Documentation
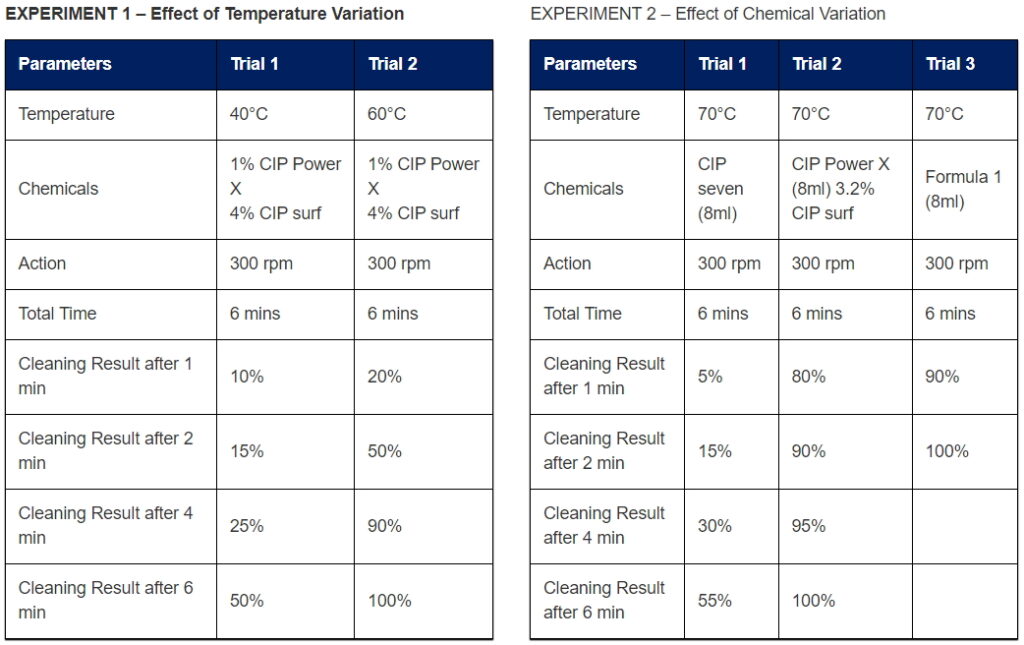
The Cleaning power is defined by TACT parameter.

Cleaning power is achieved by optimizing the TACT parameters. For example, If the cleaning at lower temperature (T) is required, then other parameters – Action (A), Chemical (C) & Time (T) can be adjusted to achieve desired result. The effect of changing TACT parameters can be noticed in following experiment
Selection of Right Cleaning Tools
As understood above; selection of right cleaning procedure is essential. It is important to select the right tool, i.e. right chemical and even right equipment for cleaning to achieve desired results and to comply with the regulatory requirements
Right Chemical
 There are various types of soils such as gels, oils, silicon, glycols, proteins, steroids, alcohols, sugars that may be found on the processing equipment. It is crucial to determine the type of soil to select the right detergent (chemical) for effective cleaning. For example, Alkaline cleaner will be useful in cleaning oil and greases. While citric acid cleaner will be required for cleaning mineral deposits, hard scales whereas enzymatic cleaner will be necessary for proteins and starch.
There are various types of soils such as gels, oils, silicon, glycols, proteins, steroids, alcohols, sugars that may be found on the processing equipment. It is crucial to determine the type of soil to select the right detergent (chemical) for effective cleaning. For example, Alkaline cleaner will be useful in cleaning oil and greases. While citric acid cleaner will be required for cleaning mineral deposits, hard scales whereas enzymatic cleaner will be necessary for proteins and starch.
Right Equipment
Based on the type of equipment or part that needs to be cleaned, the method and type of cleaning equipment are used. Broadly, the cleaning method is divided based on the size of equipment or part to be cleaned. i.e. CIP (Cleaning-In-Place) or COP (Cleaning-Out-of-Place).
Generally, CIP is employed for large equipment or components that cannot be taken apart for cleaning. CIP is carried out with an automated system spraying system which requires less time as it eliminates dismantling and re-assembly time when compared to COP.
COP is often used for small size equipment or smaller components that can be easily dismantled and re-assembled. COP can be carried out manually or using machines like Ultrasonic washer or Automated Pharmaceutical washers.
 a) Manual Cleaning
a) Manual Cleaning
Results of Manual cleaning are human dependent which may offer challenges in achieving repeated results and cleaning validation. It may be challenging to clean difficult-to-access area.
 b) Ultrasonic Washer
b) Ultrasonic Washer
The Ultrasonic cleaner uses high-frequency sound waves to agitate a liquid and cause cavitation, which creates tiny bubbles that loosen and remove debris from the surface. It is highly effective for eliminating traces of product and contaminant. The ultrasonic cavitation and implosion helps removing contaminant effectively especially from hard to clean areas. The ultrasonic washer is suitable for effective cleaning of small component and difficult-to-access areas. It offers a practical and economical solution for smaller quantities of parts where manual handling of the components is easy.
The ultrasonic washer is suitable for effective cleaning of small component and difficult-to-access areas. It offers a practical and economical solution for smaller quantities of parts where manual handling of the components is easy.
 c) Automated Pharmaceutical Washer
Equipment is operated through PLC-HMI, that is loaded with number of pre-configured cycles with settable parameters, suitable to use for various types of components. Precise chemical dosing volume is settable in cleaning program. All the data are recorded, and system is compliant to 21CFR Part 11. Cleaning program consists of various phases such as Pre-washing, Washing, Neutralization, Rinsing, Drying and Cooling. It offers facility of measuring online cleaning results which can be validated through sampling arrangement.
c) Automated Pharmaceutical Washer
Equipment is operated through PLC-HMI, that is loaded with number of pre-configured cycles with settable parameters, suitable to use for various types of components. Precise chemical dosing volume is settable in cleaning program. All the data are recorded, and system is compliant to 21CFR Part 11. Cleaning program consists of various phases such as Pre-washing, Washing, Neutralization, Rinsing, Drying and Cooling. It offers facility of measuring online cleaning results which can be validated through sampling arrangement.
Automated Washer offers an effective, efficient and validated cleaning solution for wide variety of component cleaning. It is
suitable for critical cleaning application and handling large quantities of components for cleaning. It helps to achieve repeatable & reproducible results with cleaning validation
Cleaning Validation

Cleaning validation is the process of providing documented evidence that the cleaning method employed within a facility consistently control potential carryover of products, intermediates, impurities, cleaning agent, and extraneous material into subsequent product to a level which below predetermined levels. Cleaning validation is to verify the effectiveness of cleaning procedure in removing the potential contaminant in pharmaceutical processing.
References:
- Borer Chemie
- Regulatory aspects of cleaning and cleaning validation in Active Pharmaceutical ingredients – Debaje Priyanka D., Chhabra Gurmeet S., GujarathiNayan, Jadhav Anil
Subscribe to our Newsletter
Stay tuned with Industry updates
| Thank you for Signing Up |

