
NEWSLETTER
ISSUE
Issue Jan to Mar, 2023 Volume 21
LATEST INNOVATIONS
The P-O-U system (Point-of-Use cooler)
One of the most critical requirements of any pharmaceutical manufacturing facility is to maintain sanitization and ensure zero microbial growth throughout the sterile manufacturing process.
The P-O-U system (Point-of-Use cooler) is a critical bridge between the water for injection (WFI) generation system and the user. Sanitization is maintained by ensuring the circulation of WFI in a loop at a velocity of 2.5 m/sec and at an elevated temperature of 85 to 90° centigrade. When the process demands, the WFI is cooled at Point-Of-Use. The P-O-U ensures system integrity to maintain product quality and simultaneously fulfil the user requirement of quick delivery and convenience.
Pharmalab’s P-O-U system (T-N-T type), is built to instantaneously dispense quality product with desired output at user convenience. The specially designed sanitary Tube-in-Tube heat exchanger meets the pharmaceutical industry’s high-quality requirements and hygienic standards. Notably, the compact heat exchanger facilitates instant cooling using optimum energy, thereby imparting high operational efficiency.

Design features
- No internal welds to avoid cross contamination of sanitary side and service fluid
- Single/constant dispensing flow rates (50-500 LPH)
- Single/constant dispensing temperature
- Zero dead leg provision
- Fully drainable system
- Quick response heat exchanger
Pharmalab POU system is designed to form a small parallel loop when connected to main WFI loop. This is done by tapping inlet and out (with the flow control valve in between) on the main loop. Using the advanced POU cooling system, the hot WFI is circulated continuously through small parallel loop, consisting of a heat exchanger, to maintain sterility. The specially designed heat exchanger facilitates instant cooling of WFI whenever required.
The POU not only offers a solution to sanitization but also serves as a cost-effective technology that ensures savings with respect to time and energy.
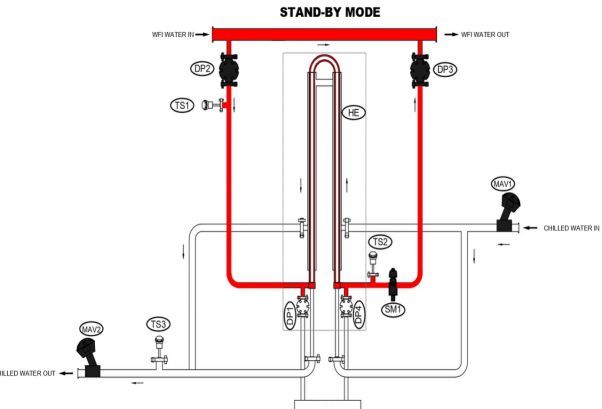
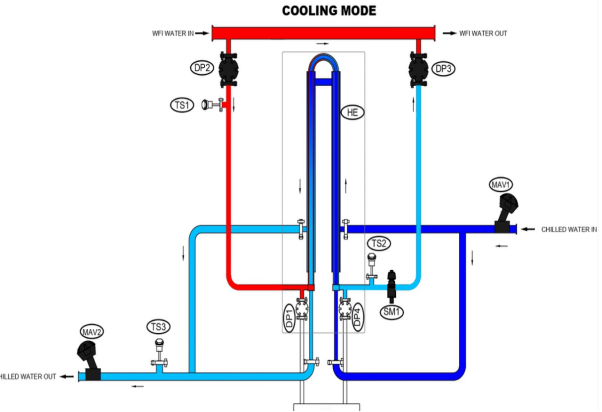
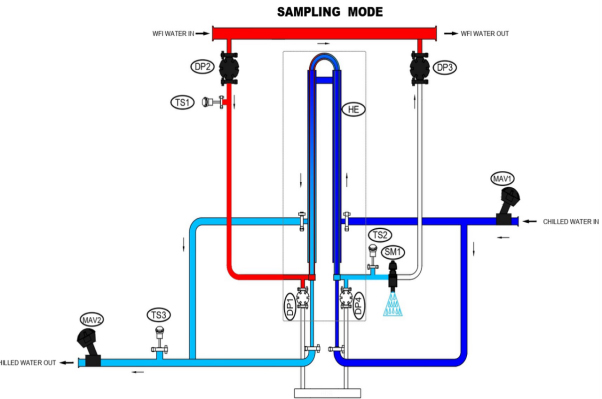
The system is compact, completely validated and constructed to fulfil ASME BPE requirements. In addition to the manual system, it offers user convenience with automated operation through HMI-PLC and possibility of remote access. Complete documentation adhering to the demanding pharmaceutical regulatory can also be provided.
For more details contact Pharmalab on pharmalab@pharmalab.com
Subscribe to our Newsletter
Stay tuned with Industry updates
| Thank you for Signing Up |

